Diagnose
SARS-CoV-2 Antigen Rapid Test Cassette
For professional use. Detect COVID-19 from one swab sample
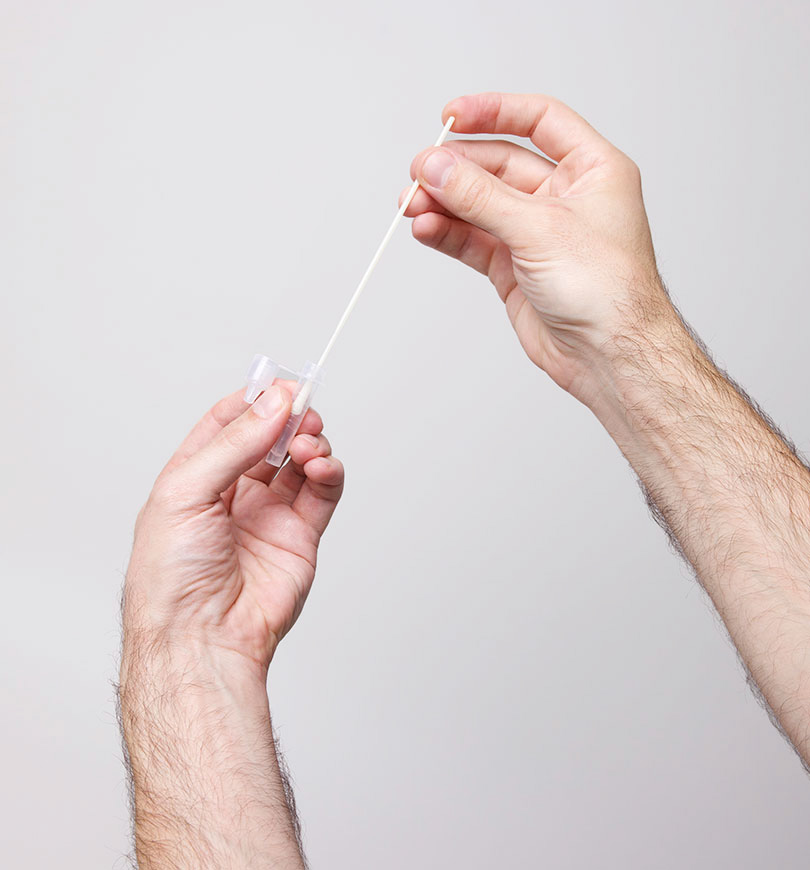
SureScreen Diagnostic’s SARS-CoV-2 Antigen Rapid Test Cassette (Nasal Swab) is a lateral flow chromatographic immunoassay single-use test kit intended for the qualitative detection of SARS-CoV-2 Antigens from anterior nasal swab specimens.
For professional use only
Key Benefits
CTDA approved
Cost saving over lab methods
Results in 10 minutes
Shallow nasal swab (Anterior Nares)
Point of care triage screening tool
Approved & Certified By



Your words about us
Join our community
Stay up to date with all of SureScreen's latest news, products and services.